Professionally, I write safety data sheets, so, my curiosity peaked when I saw two pictograms on the Iron X label! I had to know what was behind the Corrosive and Warning
Is Iron X dangerous? The Iron X safety data sheet (SDS) lists the following hazards: May be corrosive to metals; Harmful if swallowed; May cause an allergic skin reaction; and Causes serious eye damage. The source of these hazards is revealed in the Iron X ingredients listed below.

Iron X is a chemical. All chemicals have specific hazards that could make them hazardous to use. The Iron X SDS (Safety Data Sheet) explains that the primary concern is skin/eye exposure. It is also corrosive to metals; harmful if swallowed; may cause an allergic skin reaction, and causes serious eye damage. The Iron X SDS also list a number of precautions that allow you to use it safely. Those hazards and precautions will be explained in this article.
Hopefully this does not scare you. Even water is a toxic chemical if you drink enough of it. For example, consuming about 6 liters of water to kill a 165-pound person, according to the American Chemistry Society. Many of your common household cleaners can be hazardous if used improperly.
If you are in a rush, I’ll get right to the point. The Iron X SDS lists the following hazard statements:
>>>H290 May be corrosive to metals.
>>>H302 Harmful if swallowed.
>>>H317 May cause an allergic skin reaction.
>>>H318 Causes serious eye damage.
How do you protect yourself from the hazards of Iron X? Follow the Precautionary Statements listed on the Iron X SDS. The Precautionary Statements include:
P101 If medical advice is needed, have product container or label at hand.
P102 Keep out of reach of children.
P280 Wear protective gloves/protective clothing/eye protection/face protection. P302+P352 IF ON SKIN: Wash with plenty of water.
P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P310 Immediately call a POISON CENTER/doctor.
P333+P313 If skin irritation or rash occurs: Get medical advice/attention.
P501 Dispose of contents/container to in accordance with official regulations
But what does that mean? What ingredients or components are triggering those hazard warnings? The information below will help you understand each of these hazard categories AND how to protect you and your car from these hazards.
The Most accurate source of Iron X Chemical Hazards.
The information below is from the Iron X Safety Data Sheet. Safety Data Sheets (formerly called Material Safety Data Sheets) follow a 16 Section format that explains the hazards and precautions necessary to work with the chemical. These Safety Data Sheets are required for all chemicals manufactured or imported into the United States, European Union, and most countries around the world. Manufacturers and importers of chemicals have a legal obligation to provide accurate information about the chemical hazards. Considering this, there is no better resource for information pertinent to the chemical hazards of Iron X than the Iron X Safety Data Sheet.

Consider the source… Do I know what I’m talking about?
I work with Safety Data Sheets nearly every day. Understanding chemical hazards is part of my job. I am very familiar with the Global Harmonized System Purple Book, that is used globally to classify chemical hazard categories. I actually write Safety Data Sheets (hundreds per year). I understand how to use the information on a Safety Data Sheet and translate it into safe work practices for my fellow employees. In this article, I’ll interpret the SDS and share my perspective on the Iron X hazards and methods to use it safely.
What else can we learn from a SDS?
Companies may also share other information about the chemical in the transportation, and environmental sections. These sections are not mandatory in the United States. (However, nearly all manufacturers and importers do share this information.) These sections lead the SDS authors to share a bit more information about the chemical.
Can I trust a Safety Data Sheet from another country?
The United Nations developed the Global Harmonized System for categorizing hazards. This GHS system also defines exactly what information needs to be shared by the manufacturer or importer. Companies have a legal obligation in nearly every country to disclose the chemical hazards and safe handling requirements. For example, the Iron X complies with SDS Regulation (EC) No. 19072006. Specifically, a European Regulation: Regulation (EC) No 1907/2006 – Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)
of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and establishing a European Chemicals Agency
The Objective of the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and establishing a European Chemicals Agency is:
The purpose of this regulation is to ensure a high level of protection of human health and the environment. It shall apply without prejudice to Community workplace and environmental legislation. There are exemptions on medical, veterinary, alimentary and cosmetic products, polymers and some on-site isolated intermediates.
So, what does the SDS list as the chemical hazards of Iron X?
Hazard statements
H290 May be corrosive to metals.
H302 Harmful if swallowed.
H317 May cause an allergic skin reaction.
H318 Causes serious eye damage.
The Iron X SDS signal word is DANGER!
What does that mean? The Global Harmonized has two signal words: Danger and Warning. GHS had a third signal word at one point (Caution) but they didn’t think people would recognize the distinction between Caution and Warning. The Danger signal word indicates that one or more of the hazard classes are among the more serious hazard class or classes.
What exactly makes Iron X hazardous?
What hazardous components are triggering these significant hazard warnings? SECTION 2: Hazards identification actually tells us which individual components trigger the hazard warnings on Iron X:
Hazard components for labelling
ammonium mercaptoacetate
Alcohols, C10-16, ethoxylated, sulfates, sodium salts
piperonal
The SDS includes Section 3: Hazardous Components. This section of the SDS is very telling! It includes the three hazardous components of Iron X. It also lists how much of Iron X is made up of this material. AND, the hazards of each component! This section of the SDS explains that Iron X is made up of the following amount of chemicals:
25-30% Ammonium mercaptoacetate
5 – 10% Alcohols, C10-16, ethoxylated, sulfates, sodium salts
<1% piperonal
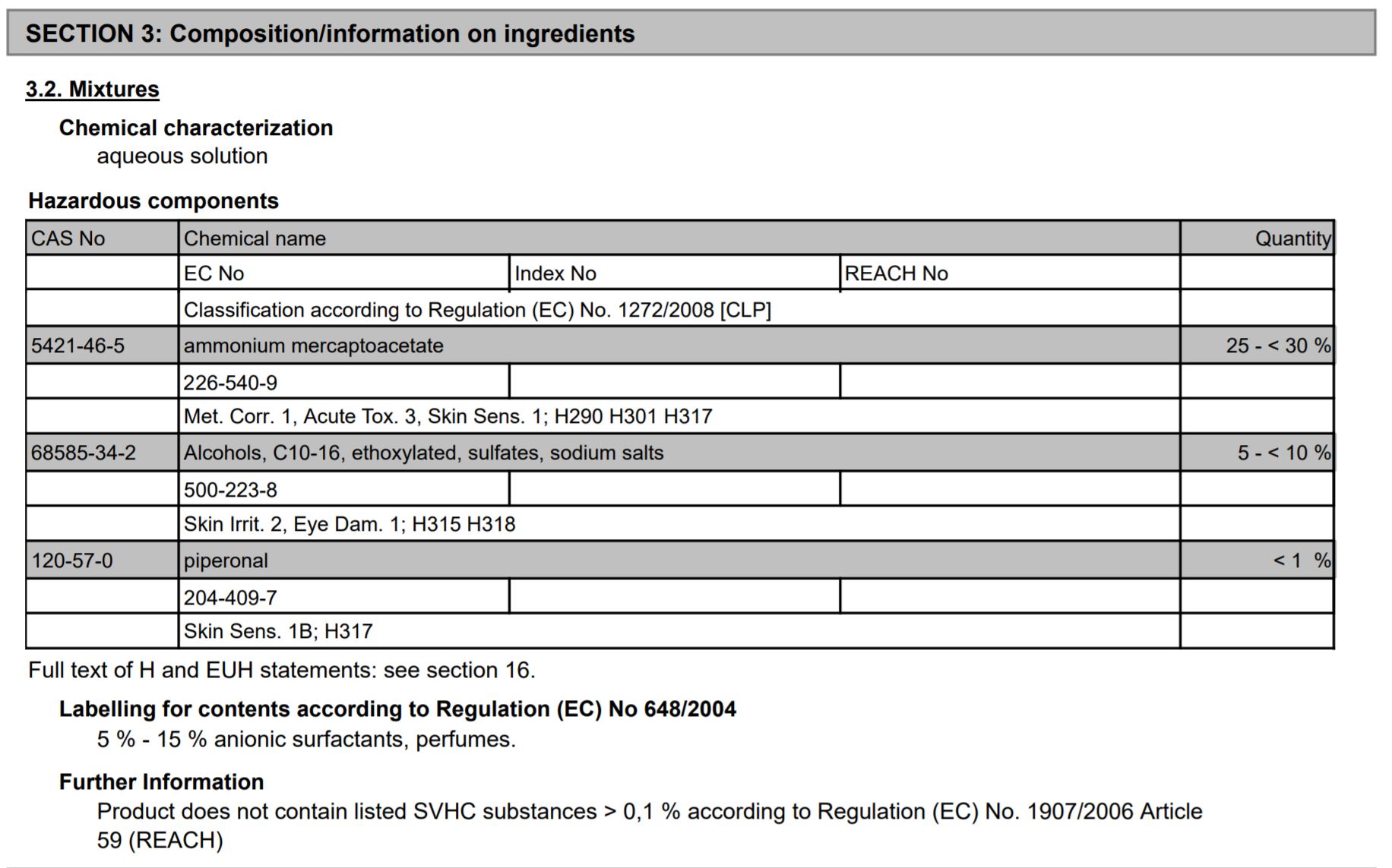
There it is! The secret formula! You’ve been wondering what’s in Iron X, now you know! This information was hidden in plain sign on the SDS. Why would CarPro share “the secret sauce?” Because all companies have a legal obligation to state the hazardous components of a mixture. Companies can state that an ingredient is “proprietary” or “a trade secret”, but they still need to list the hazards of the proprietary components. Considering that CarPro has not listed any of these ingredients as “proprietary”, there’s a pretty good chance that these hazardous components are included in every fallout remover available on the market.
If only Plankton could find the Crabby Patty Safety Data Sheet!
Let’s take a look at each of these components individually. This is where it gets really interesting! What component makes Iron X hazardous? The next section will be broken down into three sections as we explore the three components of Iron X:
Ammonium mercaptoacetate (which makes up 25-30% of Iron X)
Alcohols, C10-16, ethoxylated, sulfates, sodium salts (which makes up 5 – 10% of Iron X)
Piperonal (which makes up <1% of Iron X)
Ammonium mercaptoacetate (which makes up 25 – 30% of Iron X)
This chemical is likely the source of the bad smell. The second part of the chemical name begins with mercapt… This material is a mercaptan, which is the source of the “rotten egg” odor of Iron X. Mercaptans have this distinct “rotten egg” odor and are products of decomposition of organic material. Mercaptans are added to propane and natural gas so that we can smell leaks in these systems.
Not only is ammonium mercaptoacetate the source of the odor, it is also the source of the nasty hazard classes listed on the SDS. The Iron X SDS lists the following hazards for Ammonium mercaptoacetate (Hazard codes are listed next to each chemical hazard):
Metal Corrosive 1, (H290 May be corrosive to metals.)
Acute Toxicity 3, (H301 Toxic if swallowed.)
Skin Sensitizer 1, and (H317 May cause an allergic skin reaction.)
When I’m trying to gain perspective on the degree of hazard of a chemical, I use the ECHA database. People who write Safety Data Sheets routinely use the ECHA database. However, like all sources of information, you need to use the information “with a grain of salt”. The ECHA database summarizes the hazard categories of each chemical as it was reported to the ECHA database by European manufacturers and end users of chemicals. So, the information is only as good as the person at each company who contributed the information to the ECHA database.
The following information is from the European Chemical Agency website. This is the description provided for Ammonium mercaptoacetate:
Danger! According to the classification provided by companies to ECHA in REACH registrations this substance is toxic if swallowed, may be corrosive to metals and may cause an allergic skin reaction.
These hazard categories include: Met. Corr. 1, Acute Tox. 3, Skin Sens. 1; H290 H301 H317
The ECHA Database hazard categories matched the hazards of ammonium mercaptoacetate listed on the Iron X SDS. This tells me that CarPro has honestly shared the chemical hazards of Iron X on the Safety Data Sheet (SDS).
The Iron X SDS lists the following hazards for Ammonium mercaptoacetate (Hazard codes are listed next to each chemical hazard):
Metal Corrosive 1, (H290 May be corrosive to metals.)
Acute Toxicity 3, (H301 Toxic if swallowed.)
Skin Sensitizer 1, and (H317 May cause an allergic skin reaction.)
Let’s take a closer look at each of these hazard categories. Stopping here would be like describing a car as a “fast car”. How is it fast? Fast compared to a scooter? Drag race fast? Looks fast standing still? It’s important to understand the degree of the hazard before drawing any conclusions:
GHS describes the nature and severity of a chemical hazard by hazard class and hazard category:
Hazard Class Hazard Category
Metal Corrosive 1
Acute Toxicity 3
Skin Sensitizer 1
First: Metal Corrosive 1, (H290 May be corrosive to metals.)
When I saw Metal Corrosive, listed on the SDS, my first reaction was: “Could this be bad for my car and paint?” Perhaps you are thinking the same thing. So, let’s understand this hazard category:
What defines “Corrosive to Metals”?
Chapter 2.16 of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), Third revised edition, is dedicated to defining the Corrosive to Metals Hazard Category.
“2.16.1 defines “Corrosive to Metals as: “A substance or a mixture which is corrosive to metals is a substance or mixture which by chemical action will materially damage, or even destroy, metals”
That sounds pretty ugly to me. I can’t believe that I’d intentionally spray this onto my painted car! Ugh! All kinds of thoughts are racing through my head! How many lawsuits does CarPro deal with each year? Why did I buy this stuff? But the most important thought is: what classifies a material as corrosive to metal?
Table 2.16.1 is the classification criteria for Corrosive to Metal. Actually, the Table 2.16.1 is called “Criteria for substances and mixtures corrosive to metal. The criteria states:
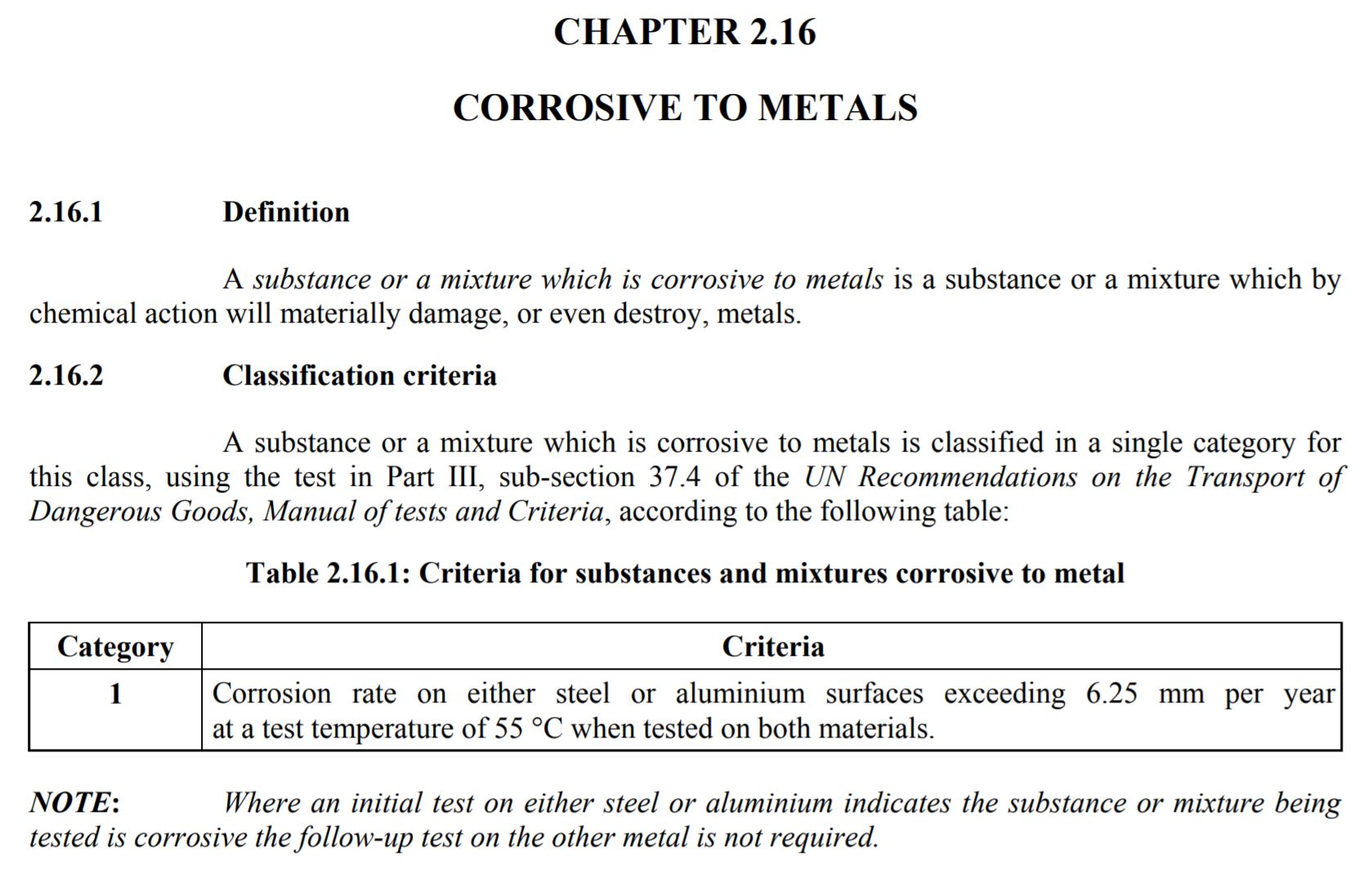
“Corrosion rate on either steel or aluminum surfaces exceeding 6.25 mm per year at a test temperature of 55 degrees Centigrade when tested on both materials.”
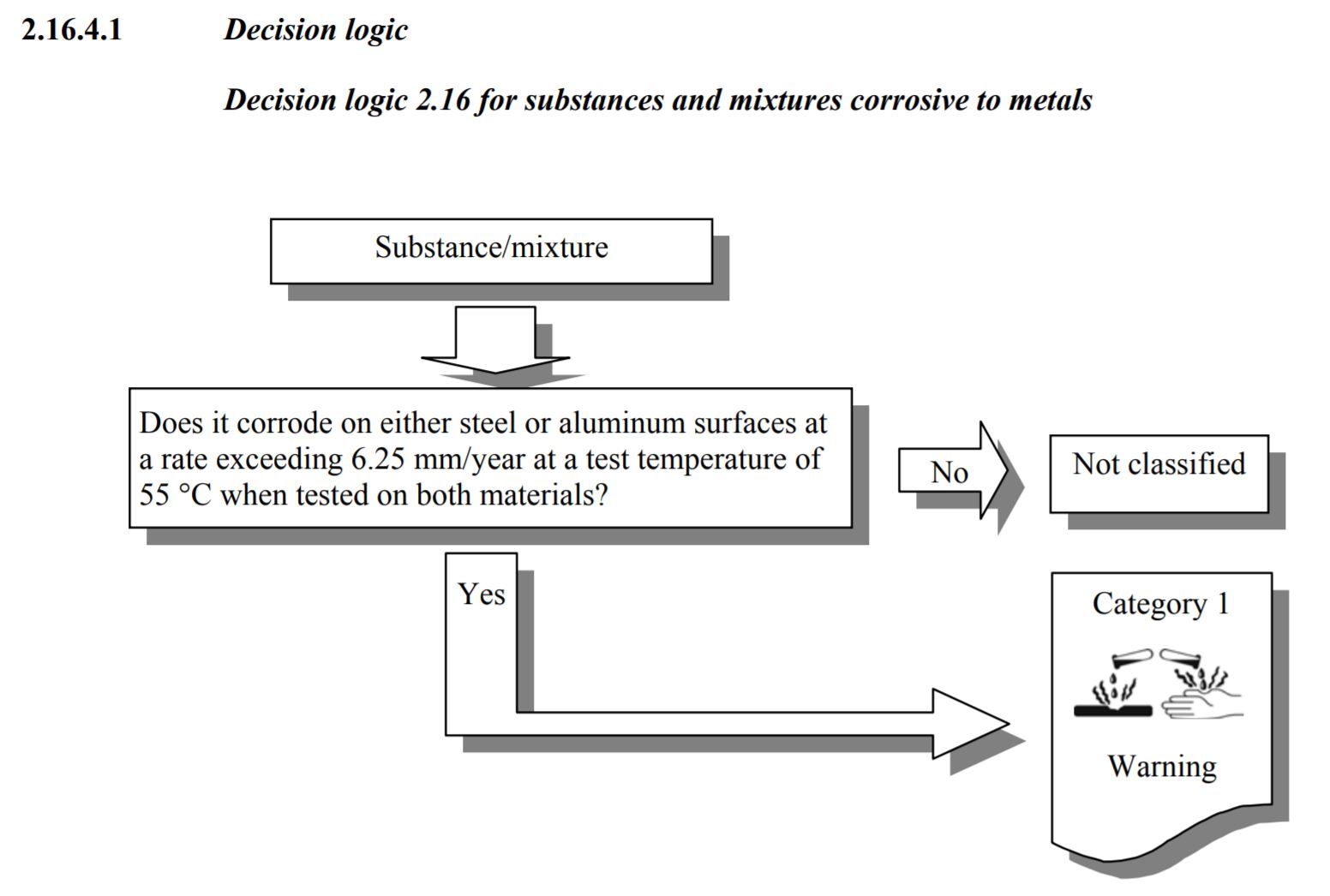
Source: United Nations Global Harmonized System Purple Book, Revision 3, Section 2.16
That definition changes my perspective significantly. Spaying Iron X onto my paint for 3-5 minutes is a much shorter time frame of one full year. Also, the temperature when I apply Iron X is less than 55 degrees Centigrade (131 degrees Fahrenheit.) Also, it is much easier to corrode unprotected (unpainted) steel. For example, the steel rotors I took off my wife’s car had a layer of rust after the first rain. Aluminum is a soft metal and quite easy to corrode.
I feel much better about spraying Iron X onto my paint after reading the criteria of Corrosive to Metal. After all, I did buy Iron X to dissolve the ferrous (iron) particles from my paint. It would make sense to me that Iron X would corrode the ferrous particles enough to get them to loosen their hold on the car’s paint.
Second: Acute Toxicity 3, (H301 Toxic if swallowed.)
The Global Harmonized System defines the hazard class “Acute Toxicity” into five degrees of hazard categories. 1 is the most dangerous hazard category. 5 is the least hazardous Acute Toxicity hazard category. Ammonium mercaptoacetate is classified as a hazard category of 3. This means that the lethal dose for 50% of the tested population (LD50) is 300 milligrams per kilogram of body weight. For example, if a group of 221 pound (100 kilogram) people were to drink 3,000 mg of Ammonium mercaptoacetate, then, 50% of the group would die. 3,000 milligrams equals about 0.003 liters. So, could you die from drinking Iron X (or other materials with Ammonium mercaptoacetate)? The answer is yes.
I know that the cherry scent may make the material it bit more palatable. But, come on….this stuff smells nasty. I don’t see you adding this as a mixer to your favorite cocktail by accident. However, I would recommend that you keep this material out of reach from children and pets.
Not everything located in the cabinet under the kitchen sink has this acute toxicity hazard warning. The material is safe to use as directed, but, not to ingest.
Third: Skin Sensitizer 1, and (H317 May cause an allergic skin reaction.)
What is a skin sensitizer?
The United Nations, Global Harmonized System, Revision 3 defines Skin Sensitizer as:
Section 3.4.2.2.1.1 states: Skin sensitizers shall be classified as GHS hazard Category 1 where sub-categorization is not required by a competent authority or where data are not sufficient for sub-categorization”
Section 3.4.2.2.1.2 states: Where data are sufficient and where required by a competent authority, a refined evaluation according to 3.4.2.2.1.3 allows the allocation of skin sensitizers into sub category 1A, strong sensitizer, or sub category 1B for other skin sensitizers.
The Globally Harmonized System for Classification and Labeling of Chemicals (GHS) defines a skin sensitizer as: Table 3.4.2 Hazard Category and sub-categories for skin sensitizers: A substance classified as a skin sensitizer if: (a) “there is evidence in humans that the substance can lead to sensitization by skin contact in a substantial number of persons,” or (b) “there are positive results from an appropriate animal test”
Contact dermatitis, including both allergic and irritant contact dermatitis, is the second most commonly reported occupational illness, accounting for 10% to 15% of all occupational diseases. Allergic contact dermatitis (ACD) – in common with other forms of allergic disease – develops in two phases. In the first phase, skin exposure of susceptible individuals to a chemical allergen causes immunological priming, which results in the acquisition of sensitization. A sensitized subject has the capacity then to mount a more accelerated secondary response to the same chemical. Thus, if exposure occurs again, at the same or a different skin site, an aggressive immune response will be elicited resulting in a local inflammatory reaction. (Source: http://alttox.org/ – non animal methods for toxicity testing.)
So, how does this apply to ammonium mercaptoacetate and Iron X. In short, keep this material off of your skin. Exposure to a sensitizer may affect you on the first, tenth, hundredth, or thousandth exposure. Bee stings are a common example of sensitizers. You may become sensitized to bee stings after a number of stings. The exposure number that triggers an effect on the body varies by person. So, how do you prevent this? Avoid the first exposure. Then, you do not need to gamble with your exposure numbers.
The most obvious exposure to your skin will be through your hands. The Iron X SDS lists butyl rubber gloves as the best protection. Don’t just buy “rubber gloves” There are several types of chemical protective glove materials. It is important to buy a pair of chemical gloves that are resistant to the particular chemical. The Iron X SDS recommends butyl rubber. Here is an Amazon link to the pair of butyl rubber gloves that I bought to to use when applying Iron X.
How is ammonium mercaptoacetate used?
The following information is from the European Chemical Agency website. This is the description provided for Ammonium mercaptoacetate:
This substance is manufactured and/or imported in the European Economic Area in 100 – 1 000 tonnes per year.
This substance is used by consumers, by professional workers (widespread uses), in formulation or re-packing, at industrial sites and in manufacturing.
Consumer Uses
This substance is used in the following products: cosmetics and personal care products, washing & cleaning products, metal surface treatment products and non-metal-surface treatment products.
Other release to the environment of this substance is likely to occur from: indoor use as processing aid and outdoor use as processing aid.
Widespread uses by professional workers
This substance is used in the following products: cosmetics and personal care products, metal surface treatment products, non-metal-surface treatment products and washing & cleaning products. This substance is used in the following areas: formulation of mixtures and/or re-packaging. Other release to the environment of this substance is likely to occur from: indoor use (e.g. machine wash liquids/detergents, automotive care products, paints and coating or adhesives, fragrances and air fresheners) and outdoor use as processing aid.
Formulation or re-packing
This substance is used in the following products: cosmetics and personal care products.
Release to the environment of this substance can occur from industrial use: formulation of mixtures.
Uses at industrial sites
This substance is used in the following products: metal surface treatment products, non-metal-surface treatment products, washing & cleaning products and cosmetics and personal care products.
This substance has an industrial use resulting in manufacture of another substance (use of intermediates).
This substance is used in the following areas: formulation of mixtures and/or re-packaging.
This substance is used for the manufacture of: chemicals.
Release to the environment of this substance can occur from industrial use: as an intermediate step in further manufacturing of another substance (use of intermediates), formulation of mixtures and in processing aids at industrial sites.
Manufacture
Release to the environment of this substance can occur from industrial use: manufacturing of the substance.
The ECHA database lists the following synonyms for Ammonium mercaptoacetate:
Ammonium Thioglycolate 59% (50% TGA)
Ammonium thioglycolate 59% (50% TGA) low odor
Ammonium Thioglycolate 59% (50% TGA) pH 6.4
Ammonium Thioglycolate 70 W (59 – 61 % TGA)
Ammonium Thioglycolate 71% (60% TGA)
Ammonoium thioglycolate 71% (60% TGA low odor
This is important! This paragraph is worth much more than chemical trivia. This is particularly important because different manufacturers of fallout removers may name the chemical differently! For example, I watched a Youtube video where the person was using a fallout remover and he kept referring to “ammonia thio”. Careful, use of different terms does not indicate different chemical in this case.
What are the hazards of Alcohols, C10-16, ethoxylated, sulfates, sodium salts (CAS #: 68585-34-2) also known as: SODIUM LAURYL ETHER SULFATE 70% which makes up 5 – 10% of Iron X)
This component, named Alcohols, C10-16, ethoxylated, sulfates, sodium salts, is described the the Environmental Protection Agency (EPA) as a Surfactant. Consumer Uses include: Laundry and dishwashing products. Industry Uses include Surface active agents. Source: The Safer Choice of the U.S. Environmental Protection Agency (EPA) helps consumers, businesses, and purchasers find products that perform and are safer for human health and the environment. URL: https://www.epa.gov/saferchoice/safer-ingredients
The hazard classes of Alcohols, C10-16, ethoxylated, sulfates, sodium salts include:
H315 (99.92%): Causes skin irritation [Warning Skin corrosion/irritation]
H318 (26.8%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H319 (71.69%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
Precautionary statements include:
P264 Wash skin thoroughly after handling.
P280 Wear protective gloves.
P302 + P352 IF ON SKIN: Wash with plenty of soap and water.
P321 Specific treatment (see supplemental first aid instructions on this label).
Response: P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P332+P313 If skin irritation occurs: Get medical advice/attention.
P337+P313 If eye irritation persists: Get medical advice/attention.
P362 Take off contaminated clothing before re-use.
These hazard warnings are not the same as the hazard warnings for the primary ingredient, ammonium mercaptoacetate. These hazard warnings are different. The hazards are for skin and eye damage. Typically, when diluted with water, the hazard levels decrease. Considering this component make up 5-10% of the total Iron X, it is safe to assume that the hazards decrease somewhat with the addition of water. This is likely why not all of these hazard warnings appear on the Iron X label or SDS.
These hazard warning reinforce the need to protect your skin and eyes through appropriate gloves and use safety glasses.
What are the hazards of piperonal (which makes up <1% of Iron X)
Piperonal is the least hazardous of the three hazardous components. The Sigma-Aldrich SDS lists only hazard for Piperonal: H15 Causes Skin Irritation. This hazard was discussed previously in the description of Alcohols, C10-16, ethoxylated, sulfates, sodium salts.
There is 5 to 10 times as much of Alcohols, C10-16, ethoxylated, sulfates, sodium salts. than piperonal. And, piperonal is less hazardous. Considering this, efforts to protect against the hazards of the other two hazardous ingredients will protect you from the hazards of piperonal.
So, how do I protect myself from the hazards of Iron X or any other fallout remover?
Short answer: Read the Safety Data Sheet.
Long answer: The primary hazards involve skin and eye contact. Iron X will cause serious eye damage. Contact with the skin will result in skin sensitization and irritation.
Protect your eyes by wearing safety glasses. If there is a splash potential, wear chemical goggles. I usually wear tight fitting safety glasses when applying Iron X. You don’t see many people on YouTube wearing safety glasses, but, after learning of the hazards of Iron X, you are probably seeing the benefits. Safety glasses or chemical goggles are a good idea for that time that the breeze blows the Iron X toward your face, or that rare time that Iron X spray ricochets off the surface and toward your face. I also have a pretty nice pair of chemical goggles that I don’t mind wearing. These are much better than the goggles you wore in high school chemistry class. These goggles have advanced significantly! Here’s a description of my goggles on Amazon.

Prevent the liquid or spray from contacting your skin. Wear gloves. The Iron X SDS recommends butyl rubber.
An alternative glove may be Nitrile rubber. The Sigma-Aldrich SDS for Ammonium mercaptoacetate recommends Nitrile rubber for accidental contact or direct contact. See the two paragraphs below from their SDS:.
Full contact Material: Nitrile rubber Minimum layer thickness: 0.11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Aldrich Z677272, Size M) Splash contact Material: Nitrile rubber Minimum layer thickness: 0.11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Aldrich Z677272, Size M)
Here’s an Amazon link that describes one brand of Nitril Rubber glove, 11 mil, as described above. You could wear this glove for 8 hours without any worry of hazardous levels of ammonium mercaptoacetate contacting your skin.